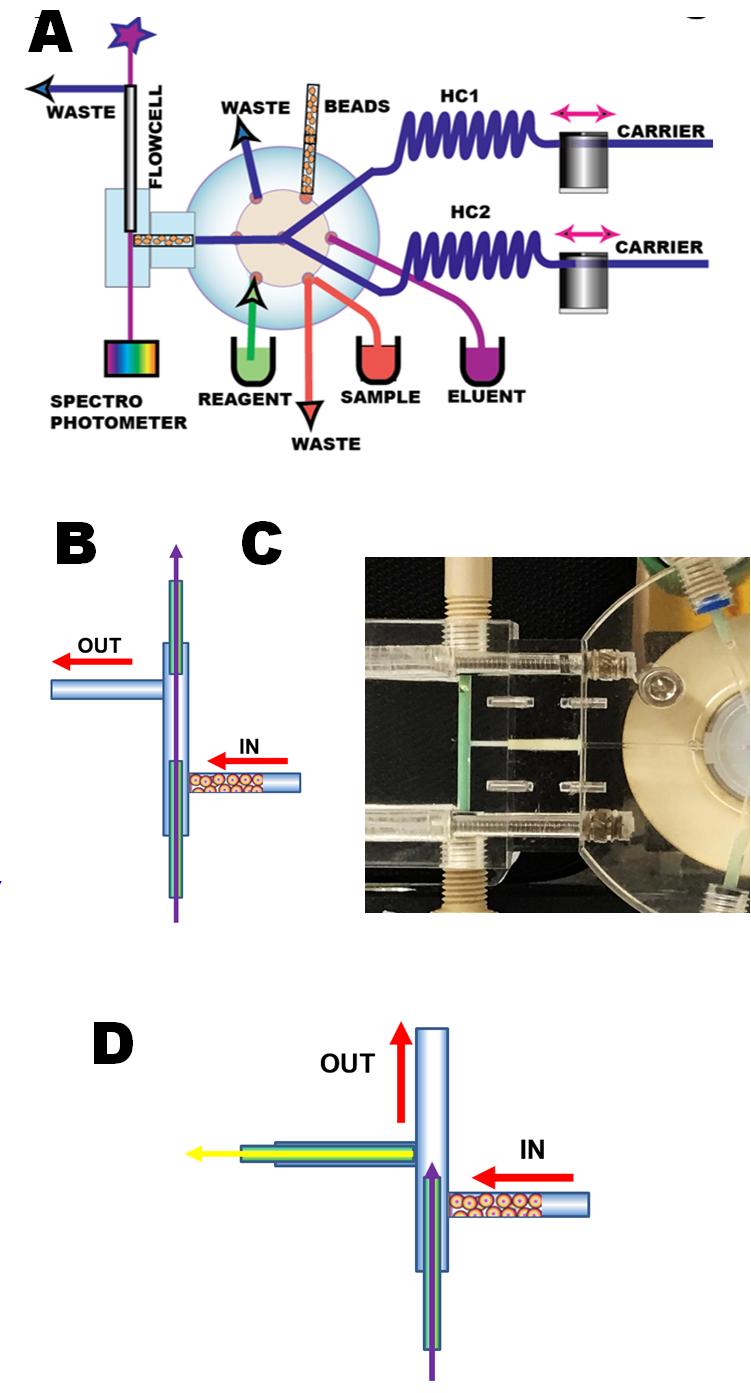
Programmable Flow Injection (pFI) manifold (A) comprises two miliGAT pumps, two holding coils (HC1,HC2), lab-on-valve module, microcolumn module and flow cell. The bead suspension is aspirated via port # 6 into the holding coil by flow reversal. After the valve is switched to port #2, the beads are packed, and retained by an optical fiber (B,C,D). Bead transport is carried out at moderate flow rates (100µL/sec), in order to transfer all beads within the selected volume of suspension through the flow channel and to pack them reproducibly into the flow cell. It was found that slower flow rates do not provide well packed microcolumn. Bead perfusion by sample solution is carried at slow flow rates (typically at 5 µL to 15 µL/sec). Bead discharge takes place as needed, by means of flow rate reversal (200 µL/sec) into the holding coil, followed by forward flush into waste after the valve switch (as shown on the next page). The unique advantage of the frit less microcolumn is a very low flow resistance that allows fast flowrates to be used for column washout and packing and ability of automated renewal of stationary phase. The particle size must be larger than 50 microns and shape should be spherical and rigid beads are preferable. Irregular silica support is not suitable. The size of microcolumn used here was 10mm long and 1.6mm I.D. Beads were kept in 25% suspension in 50% water/methanol solution.
For spectrophotometry long light path flow cell is used (A,B,C)
For fluorescence measurement flow cell is constructed with optical fibers at 90 degree configuration (D) fabricated from black polymer. The microcolumn is held in place by optical fiber.
Renewable Microcolumn in LOV Platform
3.1.6.